Al2O3 (Aluminium oxide) ceramic is the most versatile material among ceramic products. AL2O3 ceramic has high electrical insulation, high heat resistance, abrasion resistance and chemical stability, and is relatively inexpensive, so it is used in various fields. In particular, 99.7% purity AL2O3 product is widely used as a vacuum part in semiconductor chambers, and it is used to fix wafers and withstand plasma. 99.9% High purity Al2O3 (99.99%) is used in high resolution lithography miniaturization.
Aluminium oxide is also widely used as a filler in various materials, such as plastics, rubbers, paints, and ceramics. It improves the strength, hardness, and thermal properties of these materials. Moreover, aluminium oxide coatings can provide corrosion resistance and durability to surfaces in industries like aerospace and construction.

Y2O3 (Yttrium oxide) ceramics have superior plasma resistance compared to Al2O3. Y2O3 is mainly used for powder coating Al2O3 used in the semiconductor manufacturing as well as in the fields of electronics in the production of ceramic capacitors and optics as a host material for phosphors in cathode ray tubes (CRTs) and flat-panel displays.
Yttrium oxide-based compounds, such as yttria-stabilized zirconia (YSZ), are widely employed as solid-state electrolytes in fuel cells and oxygen sensors. YSZ exhibits high oxygen ion conductivity at elevated temperatures, enabling efficient ion transport in these devices.

ZrO2 (Zirconium dioxide) ceramics (MgO and Y2O3 stabilized) have a higher fracture toughness than alumina ceramics. ZrO2 is known for its exceptional mechanical properties, including high strength and fracture toughness. Its hardness is comparable to that of some metals, such as steel, and it has good resistance to wear and erosion. It is widely applied for structural ceramic parts or wear resistant parts like ceramic valve components, ceramic utility blades, ceramic grinding media, ceramic pump plungers, ceramic bearings and because of its biocompatibility in the dental and jewelry industry.
Zirconium dioxide exhibits a unique property called polymorphism, meaning it can exist in different crystal structures depending on temperature. At room temperature, zirconia is typically found in its monoclinic crystal structure. However, at higher temperatures, it can transform into either tetragonal or cubic phases.
Zirconium dioxide can serve as a solid electrolyte in solid oxide fuel cells (SOFCs). It allows the migration of oxygen ions between the electrodes, enabling the production of electricity from the combustion of fuels like hydrogen and methane.

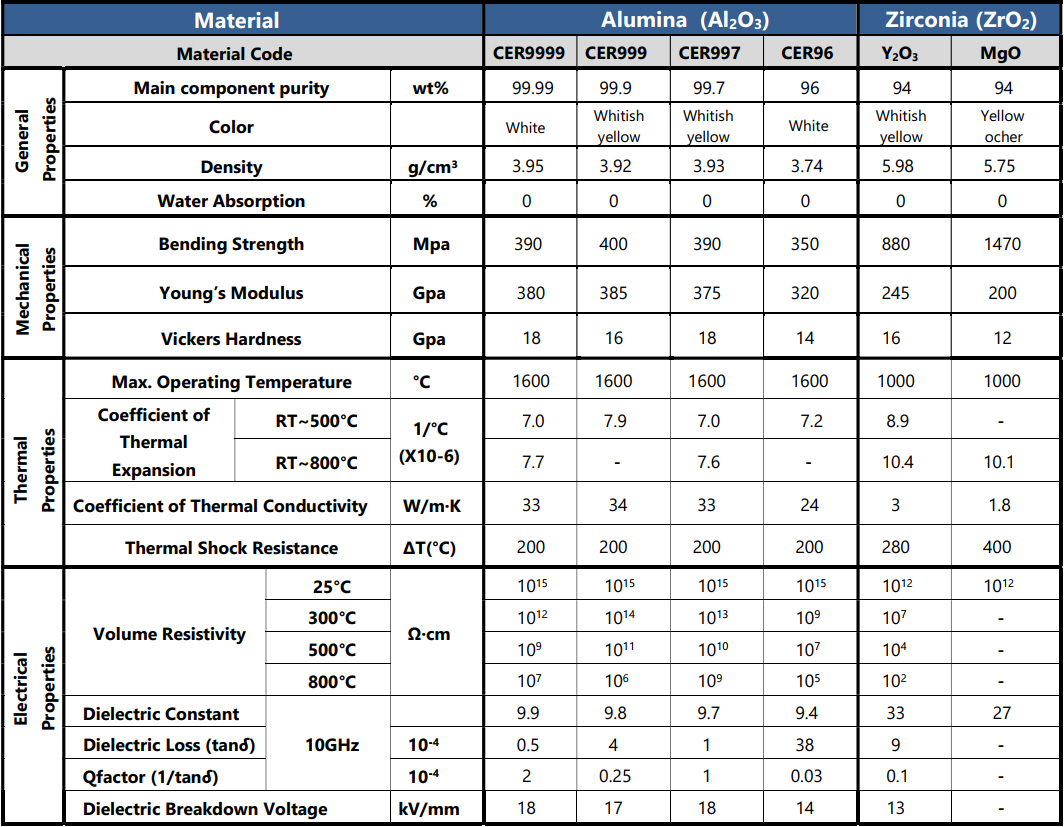
Here is an overview of the available CONvena® ceramics grades: